Calne, England/calne180
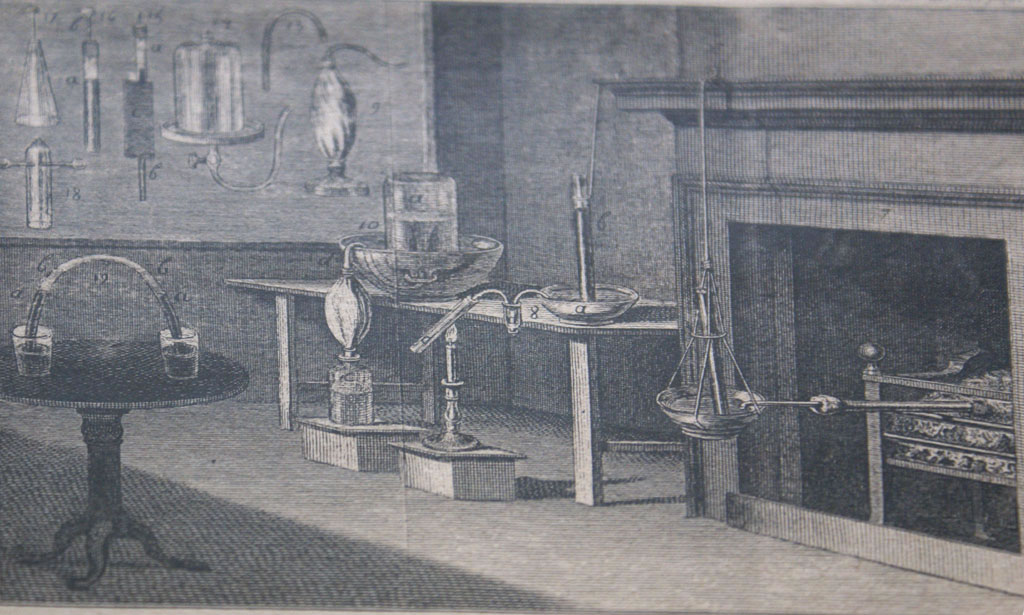
Previous | Home | NextLaboratory setup during time of Priestley. He produced ammonia by heating aqueous ammonium hydroxide solution; nitrous oxide by reacting zinc in dilute nitric acid; sulfur dioxide by heating sulfuric acid and olive oil; nitrogen dioxide by reacting nitric acid with bismuth; silicon tetrafluoride by heating fluorspar, CaF2, with sulfuric acid with subsequent exposure to glass; and oxygen by heating mercurius calcinatus per se, i.e. mercuric oxide.